The COVID-19 crisis has impacted global economies and millions of people around the world.
This guide will dive into how medical personnel can track PPE inventory to keep up with rising demand and a shortage of supplies.
In this article...
What Is a Personal Protective Equipment (PPE)?
Personal protective equipment (PPE) protects individuals from workplace hazards. Medical personnel rely on PPE to prevent disease transmissions while tending for affected patients.
Some examples of essential PPEs include:
- Surgical gloves
- Face masks
- Coveralls
- Googles
- Respirators
PPEs are categorized by the degree of protection offered: Level A, B, C, and D.
These are ranked from the highest level of protection to the lowest.
PPEs also include equipment not worn on the body. Ventilators, for instance, are a type of PPE since they prevent the spread of diseases to a certain extent.
Due to the devastating nature of the current COVID-19 crisis, PPEs from all levels are in high-demand—an unprecedented event in modern medical history.
Medical personnel and institutions are facing a worrying PPE shortage as the number of cases rise daily.
Efficient PPE inventory tracking and management is needed to ensure each medical staff receives adequate protection to carry out their work.
The Biggest PPE Inventory Management Challenges
As mentioned earlier, the supply of PPEs cannot keep up with the exponential growth of COVID-19 cases.
There are over two million confirmed cases worldwide (and growing) at the time of writing, leading to a massive surge in demand for PPEs.
Experts believe the growth won’t slow down without a vaccine, which will take at least a year to develop.
Doctors and nurses are affected the most, with many resorting to working without any PPE due to limited supply.
When an outbreak like this happens, the whole supply chain suffers and supply struggles to meet demand. In some areas, local tech companies stepped in with 3D printed parts that can substitute some PPE.
The same is happening to medical infrastructure. Hospitals are running out of ventilator and ICU beds to accommodate all patients, not just the ones affected by COVID-19.
This shortage should be resolved as soon as possible since medical personnel are critical to curbing the spread of COVID-19.
Not only are their lives endangered, people around the world will also be more vulnerable as our frontliners don’t have the resources they need to treat patients safely.
One way to alleviate this problem is to introduce better tracking systems to manage large PPE inventories.
Governments are spending big on buying PPEs from suppliers all over the world.
Without an effective tracking system, it’s difficult to distribute and manage PPE inventories while ensuring everyone’s needs are met. Some institutions could obtain more PPE than they need while others may not even receive anything.
A tracking system is necessary to keep up with ordering PPEs in the current climate.
Medical equipment like ventilators are in high demand but they have minimal supply. Medical institutions need to utilize inventory tracking systems to determine optimal reorder points so they don’t run out of PPEs in crucial periods.
This makes it easy to track and restock PPE inventories while eliminating the need for manual labor—a resource more important than money at this time.
Tools and processes should also be revamped to focus on PPE tracking and usage monitoring. Staff members should only use the right amount of resources to eliminate PPE waste.
Again, a tracking system comes in handy here since it can monitor usage, check equipment status, and track inventory levels among a variety of useful features.
Institutions can deploy the same system across different locations easily as all PPE data is stored on the cloud, eliminating the need for expensive hardware or migrations to sync information.
Medical PPE Inventory Tracking and Management: How Does It Work?
Inventory management for medical PPEs works the same as for other assets. However, extra care has to be taken to ensure PPEs are not damaged or contaminated in storage. Different PPEs also have different management criteria.
A ventilator, for example, requires more than just quantity tracking. You also need to know:
- When was it last maintained?
- What is its current status (is it working as expected?)
- Are more ventilators needed to keep up with demand?
Let’s look at how you can structure your PPE inventory management for maximum safety and efficiency.
#1 Determine Hazards and Corresponding PPEs
Any PPE program starts with determining the hazards your team faces daily.
For medical staff, this would be potential exposure to patients, hazardous chemicals, or high-risk environments.
This enables you to procure the right PPEs which is important for two things:
- you save money by buying only the equipment you need.
- you don’t take away PPEs that are essential to other healthcare professionals.
The hazard identification process should involve the entire medical team so everyone has their needs covered.
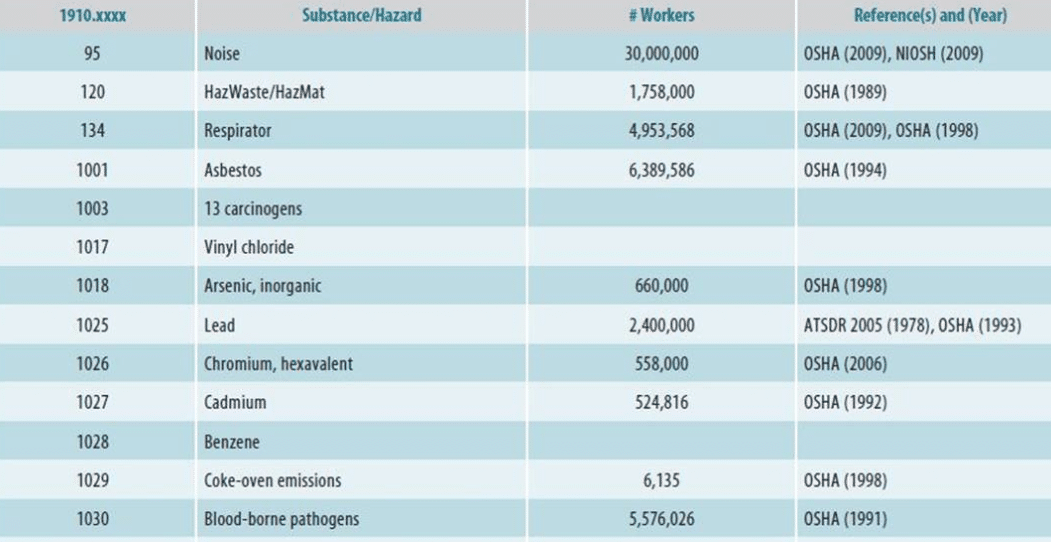
Examples of different hazards that workers are exposed to as listed by OSHA
Once the hazards are identified, it’s time to choose the best PPE for each situation. Consider these two factors when selecting a PPE for your inventory:
- Does it offer enough protection for the hazard? (refer to the PPE levels mentioned above)
- Is the PPE effective, comfortable to wear, and does not make the situation more dangerous?
The second point can be resolved with equipment testing.
PPE testing is important for healthcare institutions not only to protect staff members and patients but also to comply with regulations.
The CDC has established industry-wide guidelines for procuring medical equipment in the United States which should be similar in other jurisdictions.
Certain equipment requires additional tests and certifications to get them approved.
Respirators, for instance, need to go through a series of ‘fit tests’ before they can be used.
The CDC document linked above contains all the information you need to know about PPE testing, including guidelines for common equipment like gloves, masks, and uniforms.
#2 Source Wisely
Sourcing from the right firms is another important aspect of PPE inventory management.
You want to work with suppliers that have a track record of producing high-quality, industry-grade equipment.
Don’t cut corners here as you risk harming medical personnel and breaking healthcare regulations—both of which come with hefty penalties.
It’s best to work with reputable PPE suppliers like 3M and Honeywell if you want the best equipment in terms of safety and effectiveness.
Second-hand sourcing—including reusable PPEs—is common in the medical industry due to the high cost of brand new equipment.
When buying second-hand equipment, pay attention to the seller’s background. Getting a good deal from a local hospital is less risky than buying off an unknown source. If there are no trusted sellers, look into online medical equipment marketplaces that offer product warranties and guarantees.
Consider looking into global or nationwide initiatives to acquire PPEs if your needs cannot be met by suppliers.
The WHO and WEF-backed Pandemic Supply Chain Network (PSCN) is an example. The PSCN links private sector partners and healthcare institutions all over the world to support supply chains during pandemics. Getting involved with such initiatives can get you valuable resources to stay afloat in times of distress.
Speaking of guarantees, you should always check if a piece of equipment is FDA-approved before it goes into your inventory.
PPEs like masks, respirators, and gloves can only be sold in the United States if manufacturers adhere to the FDA’s guidelines. This requirement, also known as the Premarket Notification or 510(k) clearance, verifies if a PPE is safe and effective to be used in real-world situations by testing several factors like:
- Performance (does it work as intended?)
- Product labeling
- Tear resistance
- Sterility
Working with regulated equipment will save your institution millions from avoiding potential lawsuits down the line.
Authorities are not hesitant to dish out exorbitant fines to companies found guilty of using uncertified PPEs due to the critical nature of healthcare, as shown by million-dollar scandals in the past.
You should use a tracking system to manage your sourcing since you’re likely to make deals with multiple suppliers.
This streamlines procurement as you can use the cloud to sync purchase information with your inventory system as well as platforms like hospital information systems (HIS), eliminating mistakes that arise from human error.
#3 Storing Your Equipment
The next step after sourcing your equipment is to identify how your PPEs will be stored.
PPE storage is perhaps the most important factor to consider in your inventory management strategy. PPEs exist to protect medical staff from hazards, many of which are life-threatening, so keeping them pristine is second to none.
They should be stored as suggested by industry guidelines including avoiding sunlight, contaminants, and unsecured environments to prevent compromising PPEs.
Use an organization system to sort out your PPEs. Your colleagues should be able to find equipment without rummaging through unorganized closets.
You can use the ABC technique to separate equipment into levels based on their consumption value. Sorting by letter is another great way to create a logical storage structure for your PPEs. No matter what technique you use, make sure it eases equipment discovery, not slow it down.
An inventory tracking system is useful here as you can monitor in and out movements for PPEs easily. This helps with organization since you have clarity on where a piece of equipment is at different times.
The more organized your PPE inventory, the better your staff can work as they can use PPEs without having to guess where they are kept.
It also helps determine when a piece of equipment is running low which mitigates unexpected supply shortages.
If you’re keen to learn more about PPE storage, Alberta Health Services has a terrific document outlining how medical equipment should be stored to prevent contamination from infectious diseases.
#4 Usage Compliance
Storing PPEs the right way is just one part of the equation.
Compliance also matters if you want to get your PPE inventory tracking right. Bear in mind that PPE compliance also includes how your team members engage with equipment i.e. are they using it as mandated by healthcare regulations?
PPE usage should always be tracked the moment they leave your inventory.
This is crucial as most PPEs have a specific lifecycle that determines how long they can be used before they are no longer safe or effective. N95 masks, for instance, should only be used up to eight hours for optimal protection.
PPEs can last anywhere between 30 minutes to several hours, with higher-level equipment lying at the shorter end of the scale.
The question is, how do you achieve usage compliance in an organization with hundreds (or even thousands) of medical staff?
You should always have a recording system in place to monitor PPE usage. Every medical staff needs to record when they take a PPE out as well as how long they’re using it.
For reusable equipment, check in and check out times are also required to make it easy for future staff to use it.
In an ideal scenario, a PPE should only be used once for as long as its lifecycle to maximize protection. Equipment sharing is discouraged since individuals are less protected, not to mention the possibility of human-to-human transmissions.
However, there are situations where you are forced to reuse PPEs beyond the suggested period of usage such as the COVID-19 crisis.
In times like this, you need to maximize usage efficiency as there are insufficient supplies to cater for everyone.
PPEs can be conserved if medical staff track how long they have equipment on—like tracking reusable PPEs, for example.
With this information, institutions know when to disinfect a piece of equipment and make it available for someone else or discard it if it’s no longer usable.
An inventory tracking system helps achieve compliance by automatically collecting timestamps throughout the entire equipment lifecycle.
For example, an institution can configure its system to update stock counts and record important data the moment a PPE is taken out of storage. This improves compliance since medical staff no longer need to record usage by hand.
It also gives them more time to spend on real work—treating and caring for patients.
Since data is stored on the cloud, you can access critical equipment data anywhere, anytime no matter if you’re on your phone or laptop.
This avoids unwanted supply shortages as well since you can view stock levels round the clock. With the right inventory management system, you can track equipment usage easily to ensure PPEs are used as recommended at all times, making compliance a breeze.
#5 Medical Protective Equipment Maintenance
Protective equipment maintenance should also be a critical component of your inventory management strategy.
PPEs need to be taken care of as they are often the only protection medical staff have in hazardous situations.
A damaged full-body suit, for example, creates gaps that allow contaminants to penetrate the outfit. An individual unaware of the problem will be exposed to lethal viruses without him or her knowing anything about it until it’s too late.
It’s best to hold consistent checks to verify the quality and effectiveness of PPEs. Daily checks may seem excessive at first but some medical positions require heavy equipment checks and decontamination.
Prevention is always better than cure.
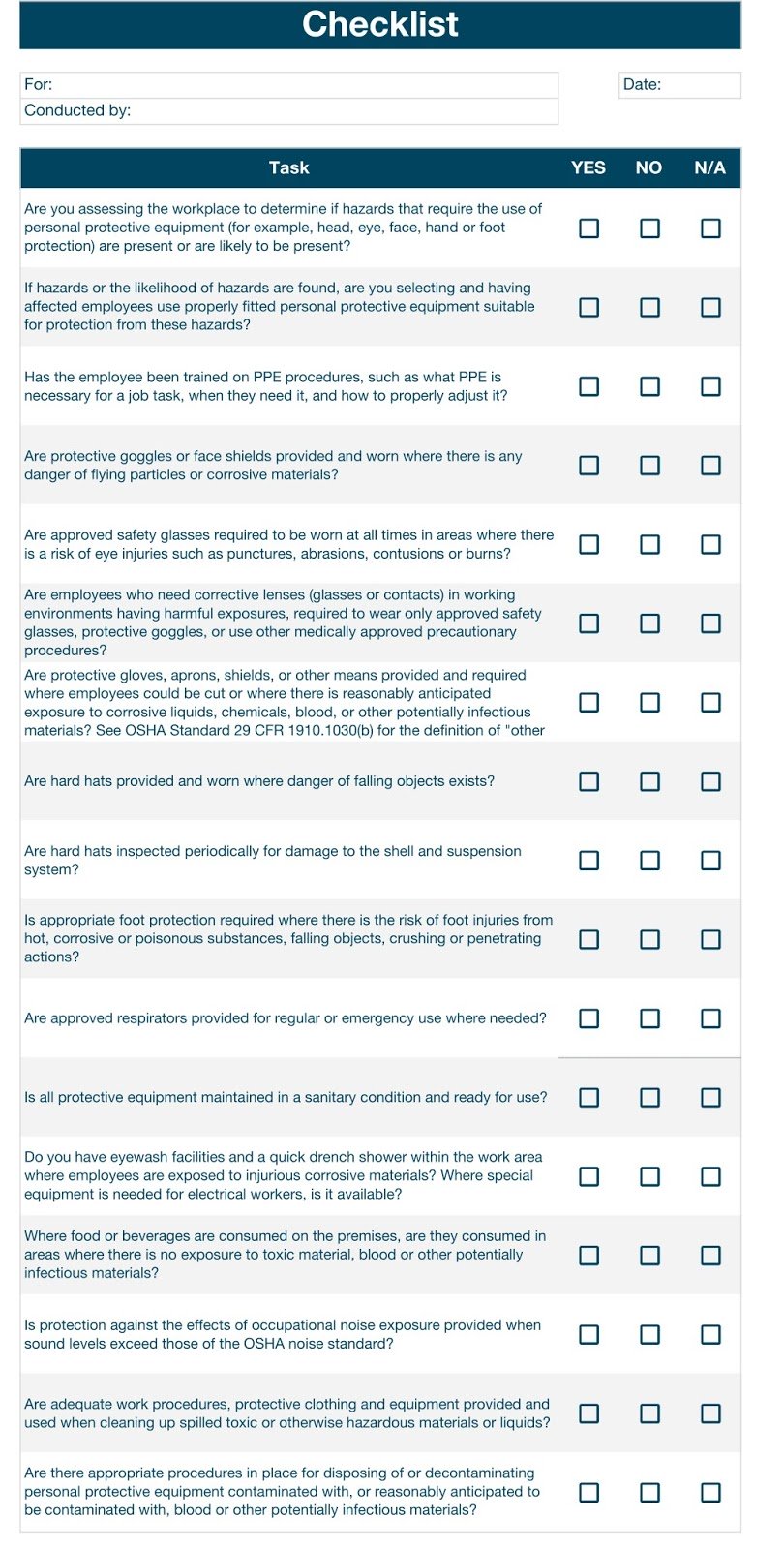
Inspection forms can be used to assess PPEs. There should be no room for leniency when inspecting medical equipment. Even if only one point fails, the PPE should be discarded or returned to the supplier if you have a warranty.
Below is a sample inspection form you can tweak to meet your institution’s needs.
There are differences when it comes to managing sterile equipment. Sterile PPEs should always be kept in their original packaging to prevent contamination.
Check if sterile packages are intact when they arrive to avoid stocking compromised PPEs. Consider working with established suppliers that have strict sterile equipment distribution policies such as the ones highlighted by the Medical Device Reprocessing Association of Ontario.
The most important step is to separate sterile equipment from other PPEs in storage. This includes using separate containers, tools, and environments when handling any sterile PPE.
An example of good practice is to have a different disposal bin to discard used sterile equipment. The better you manage your sterile equipment, the less likely it is to be damaged which lends itself to increased protection from workplace hazards.
Speaking of disposal, you should implement a waste management system to discard used PPEs safely. Used medical equipment contains traces of harmful contaminants and can expose individuals to hazards if they’re not disposed of the right way.
Viruses can last up to three days on exposed surfaces like face masks, according to various studies and researches.
Proper PPE disposal starts with establishing a doffing area for medical personnel.
A doffing area is a zone designated for the safe removal and disposal of medical PPEs.
The area should contain chemical and general waste bins to discard PPEs based on the condition of the equipment. There should also be a sink or shower nearby for medical staff to clean themselves.
The area must be decontaminated and disinfected regularly to prevent it from becoming a hotbed for diseases.
The NHS has a great video on how to remove PPE safely.
All the maintenance tasks mentioned above can be streamlined with an inventory tracking software.
For instance, you can schedule timely reminders for inspections so your PPEs are always quality-checked for safety and reliability. You can even track service histories for every piece of equipment to maintain compliance with healthcare regulations.
Make sure you track PPEs with medical-grade inventory tags. These tags are made from metal or RFID material that can work in high-temperature environments (e.g. autoclaves).
This allows them to be sterilized along with PPEs which eliminates health and compliance risks when deploying tracking tools. Check if your tags can survive sterilization before you invest in an inventory tracking system.
Track Your PPEs Safely
Using an inventory management system will do wonders for your PPE inventory especially in challenging times like the current COVID-19 crisis.
However, confirm that you have the right tools, procedures, and strategies in place to manage PPEs while maintaining compliance and eliminating safety risks.
Follow the tips mentioned above and you’ll be well on your way to a better PPE inventory management program.




